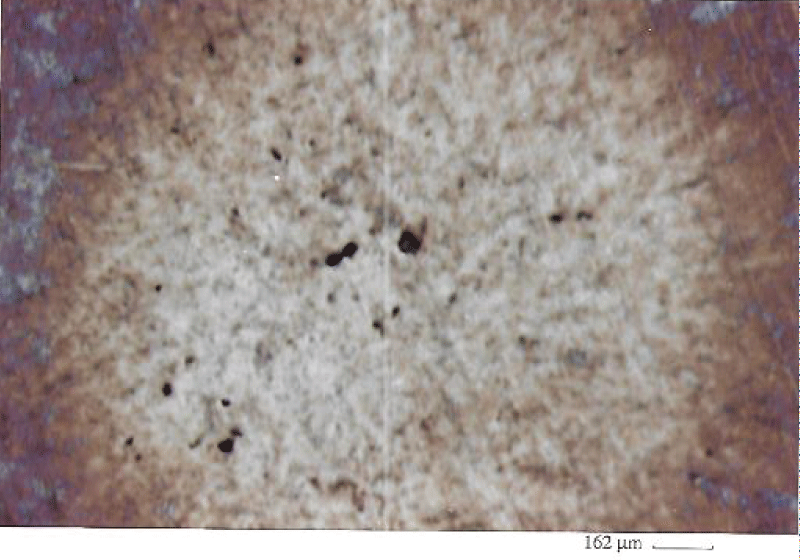
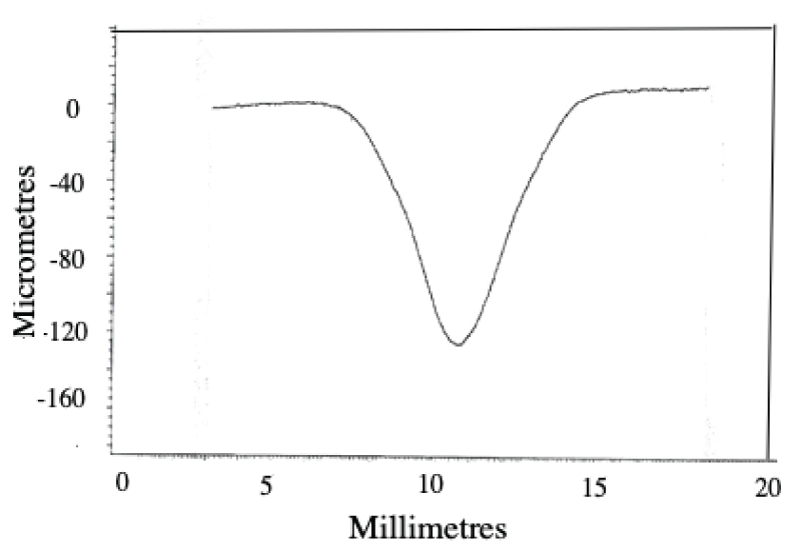
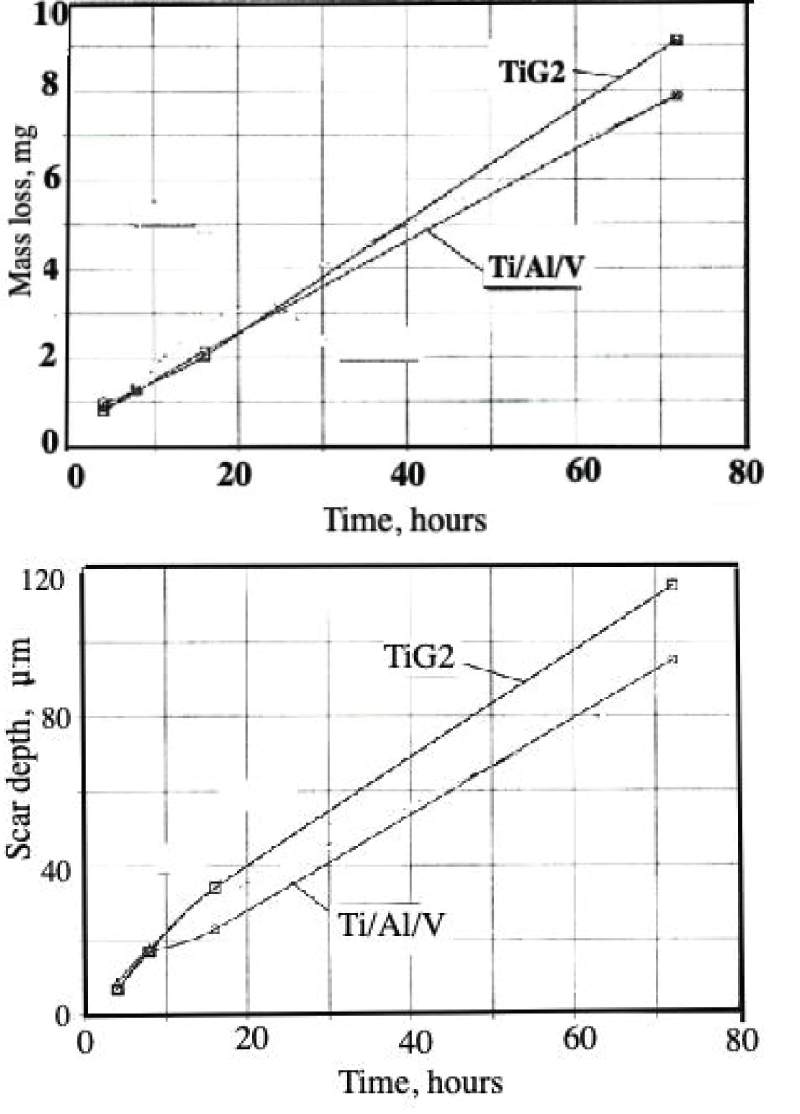
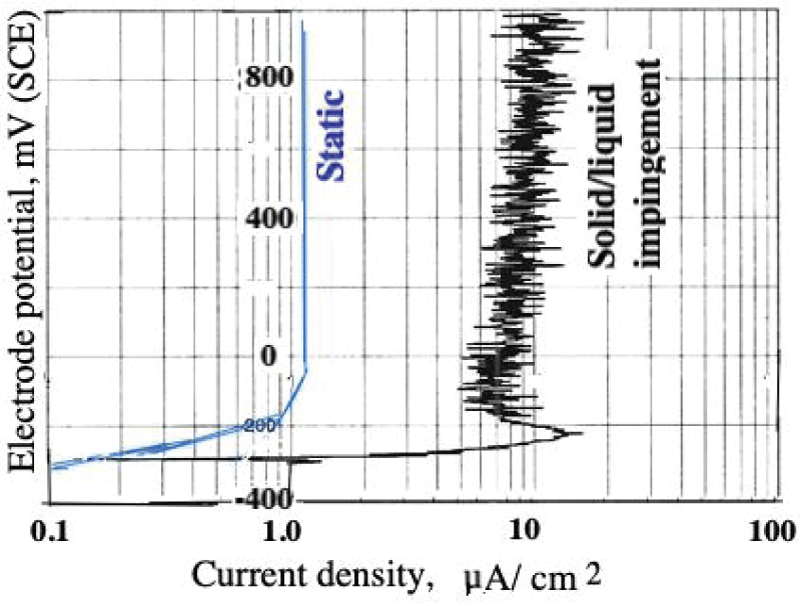
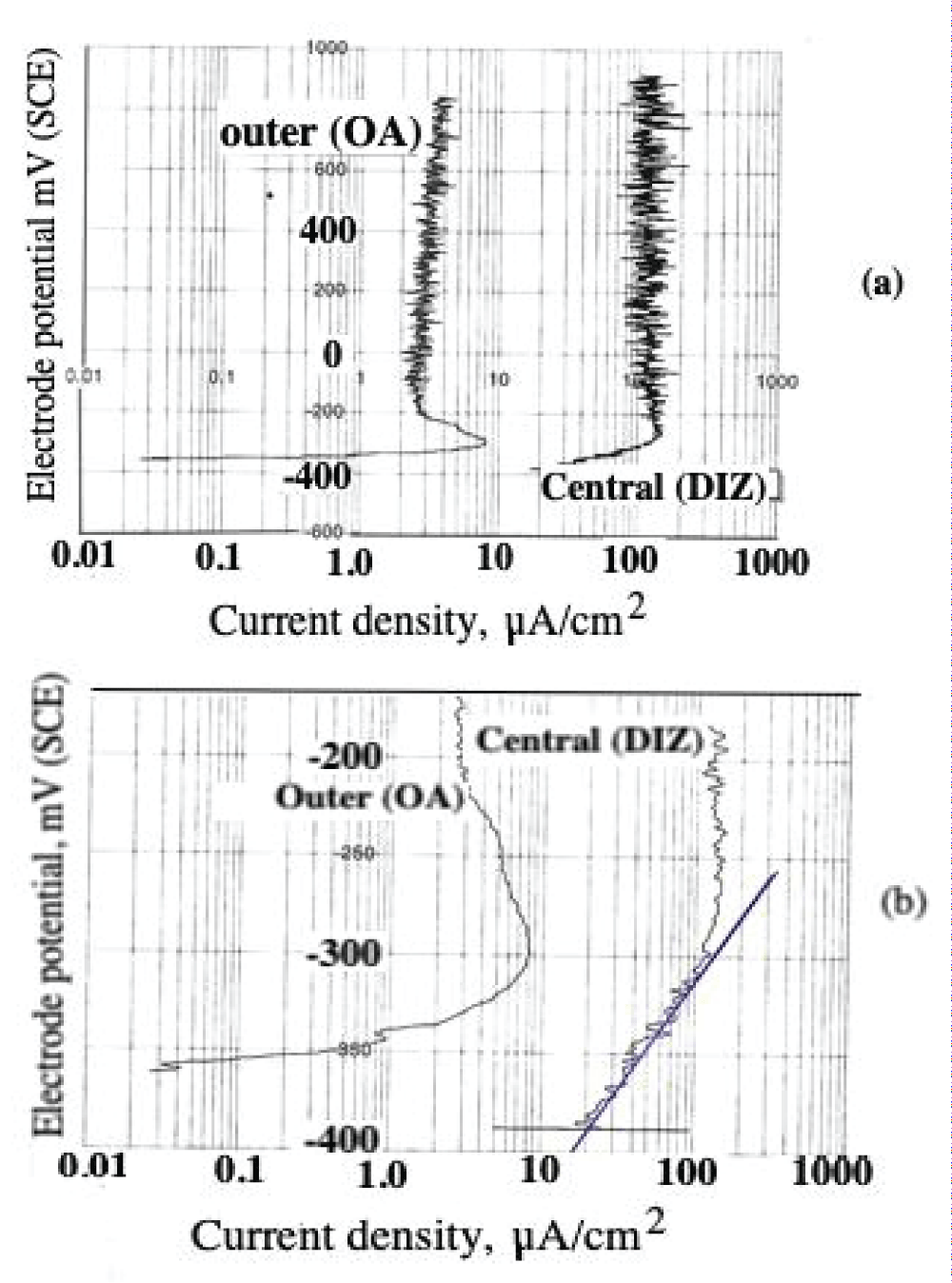
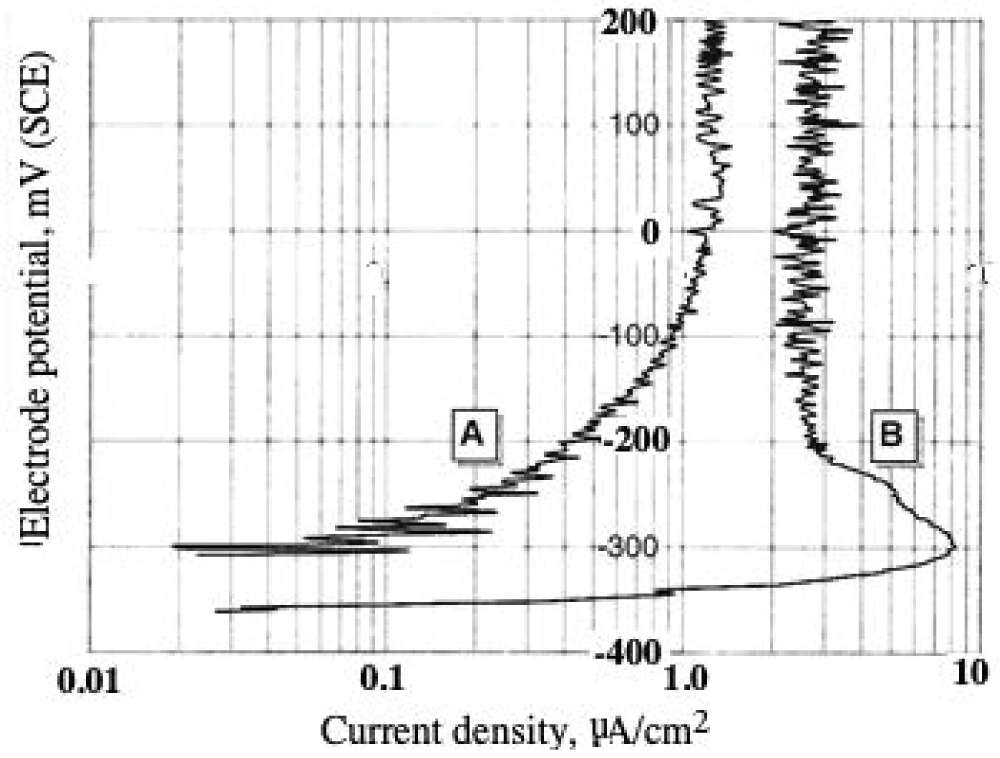
Abstract
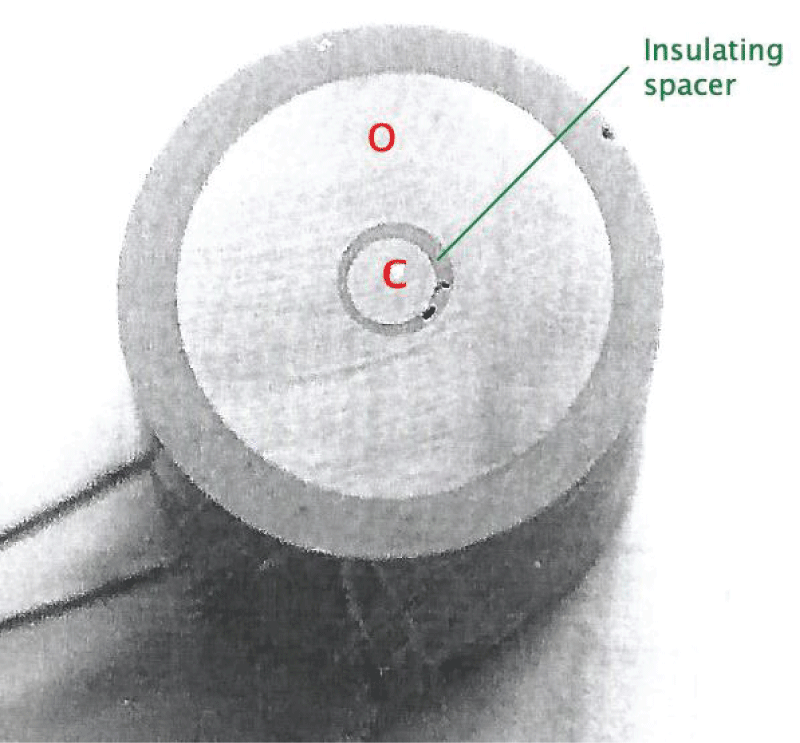
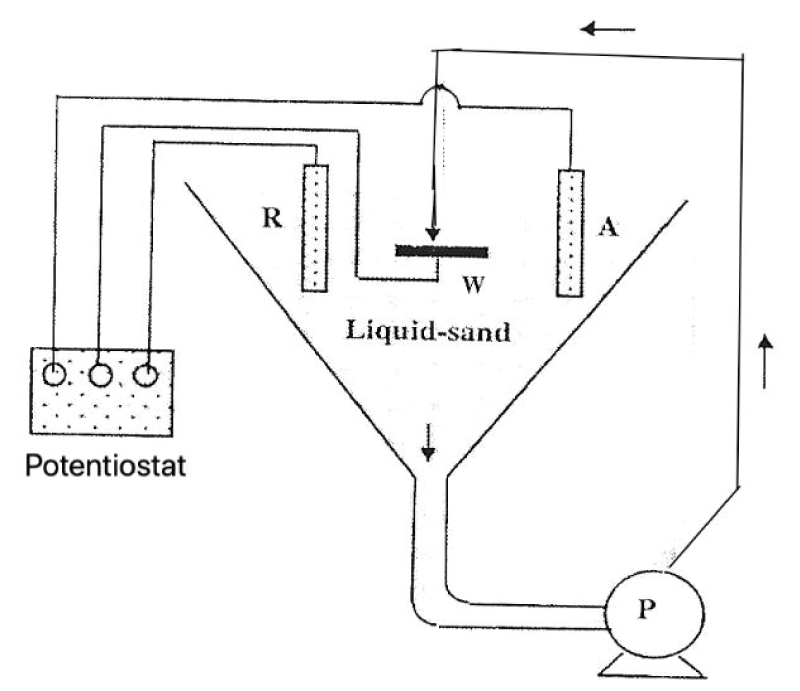
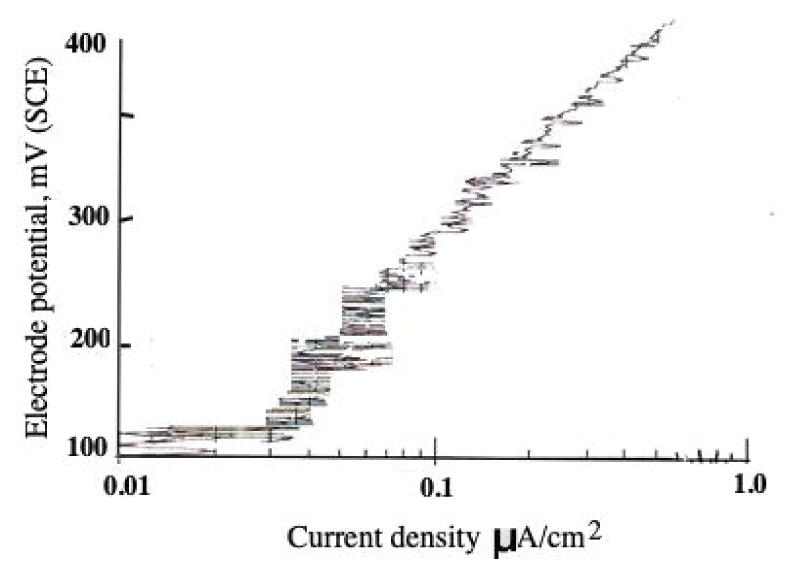
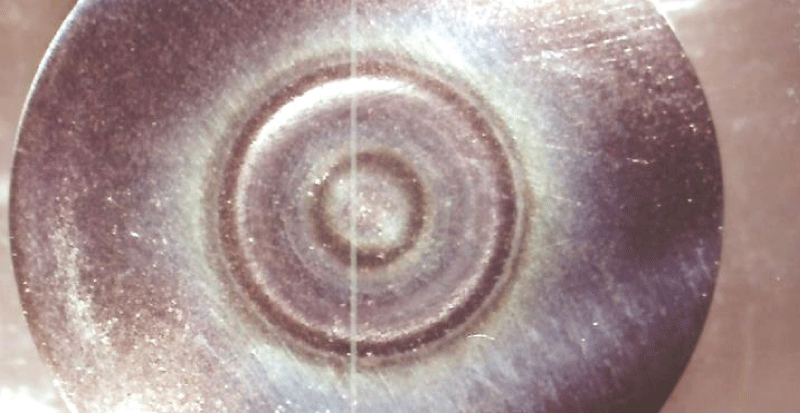
This paper describes the findings from an experimental study of the performance of commercially pure titanium and the alloy Ti/6Al/4V in high velocity 3.5% NaCl aqueous solution with and without suspended solids. The investigation involved mass loss measurements, electrochemical monitoring, surface profiling and microscopical examination. Submerged jet testing equipment was utilised using 90° impingement. The two materials exhibited excellent durability in solids-free saline water under an extremely high impingement velocity of 71 m/s at ambient temperature (18-20 °C) and 50 °C. In the presence of suspended sand over the range of 500 - 1800 mg/l, however, the durability of the materials was severely compromised under a jet velocity of about 12.6 m/s. The Ti/6Al/4V alloy demonstrated somewhat superior resistance to erosion corrosion than the commercially pure titanium. A major objective of the work, namely the quantification of the proportions of pure mechanical erosion, pure corrosion and the interactive synergy components, was accomplished. An additional feature of the research involved the adoption of an experimental methodology that facilitates the discrimination of damage between the two hydrodynamic zones (directly impinged and surrounding regions) via the use of a segmented specimen arrangement. This procedure demonstrated that, whilst the most severe damage was experienced in the zone under direct impingement from a small diameter nozzle, there was, nevertheless, a significant attack within the surrounding region where the fluid flow was at lower angles.